
Some solid KNO3 remains at the bottom of a stoppered flask containing a saturated KNO3(aq) solution at 22°C. Which statement explains why the contents of the flask are at equilibrium?The rate of dissolving is equal to the rate of crystallization. The rate of dissolving is greater than the rate of crystallization. The concentration of the solid is equal to the concentration of the solution. The concentration of the solid is greater than the concentration of the solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
Some solid KNO3 remains at the bottom of a stoppered flask containing a saturated KNO3(aq) solution...
Questions


Mathematics, 23.01.2022 20:30


Computers and Technology, 23.01.2022 20:30


Mathematics, 23.01.2022 20:30


Biology, 23.01.2022 20:30

Mathematics, 23.01.2022 20:30

Mathematics, 23.01.2022 20:30



Advanced Placement (AP), 23.01.2022 20:30


Chemistry, 23.01.2022 20:40


Mathematics, 23.01.2022 20:40



English, 23.01.2022 20:40

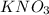 from aqueous
from aqueous 

