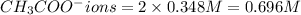In the laboratory you dissolve 14.8 g of chromium(II) acetate in a volumetric flask and add water to a total volume of 250 mL. What is the molarity of the solution? M. What is the concentration of the chromium(II) cation? M. What is the concentration of the acetate anion? M. Submit AnswerRetry Entire Group

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1


Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
In the laboratory you dissolve 14.8 g of chromium(II) acetate in a volumetric flask and add water to...
Questions



Mathematics, 02.02.2020 18:50


Mathematics, 02.02.2020 18:50


Mathematics, 02.02.2020 18:50

History, 02.02.2020 18:50


Mathematics, 02.02.2020 18:50



Mathematics, 02.02.2020 18:50





Mathematics, 02.02.2020 18:50


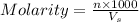
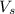 = volume of solution in ml = 150 ml
= volume of solution in ml = 150 ml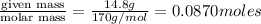
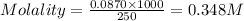
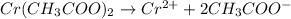
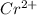 ions = 0.348 M
ions = 0.348 M