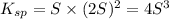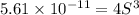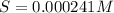Chemistry, 26.03.2020 23:46 tinasidell1972
Mg(OH)2 is a sparingly soluble compound, in this case a base, with a solubility product, Ksp, of 5.61×10−11. It is used to control the pH and provide nutrients in the biological (microbial) treatment of municipal wastewater streams. Based on the given value of the Ksp, what is the molar solubility of Mg(OH)2 in pure H2O?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
Mg(OH)2 is a sparingly soluble compound, in this case a base, with a solubility product, Ksp, of 5.6...
Questions

Mathematics, 04.09.2020 01:01

Mathematics, 04.09.2020 01:01

Mathematics, 04.09.2020 01:01

Health, 04.09.2020 01:01

Mathematics, 04.09.2020 01:01



Mathematics, 04.09.2020 01:01

Social Studies, 04.09.2020 01:01



Mathematics, 04.09.2020 01:01

Biology, 04.09.2020 01:01

History, 04.09.2020 01:01


History, 04.09.2020 01:01


Mathematics, 04.09.2020 01:01


Social Studies, 04.09.2020 01:01

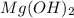 in pure water.
in pure water.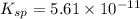
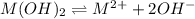
![K_{sp}=[M^{2+}][OH^-]^2](/tpl/images/0566/2963/a461b.png)