
Chemistry, 26.03.2020 23:53 melanyaguirre25
What mass of hydrogen peroxide must be used to produce 1.00 L of oxygen at 25.0°C and 1.00 ATM?
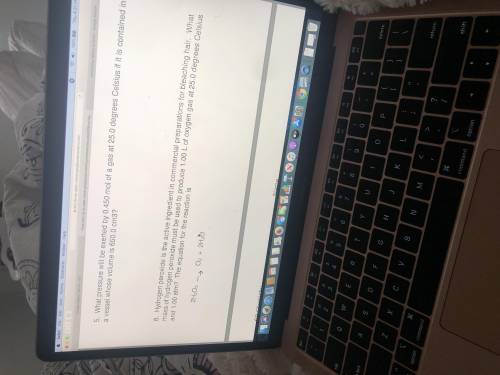

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2


Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
What mass of hydrogen peroxide must be used to produce 1.00 L of oxygen at 25.0°C and 1.00 ATM?
Questions





Mathematics, 17.10.2020 09:01




Mathematics, 17.10.2020 09:01


Computers and Technology, 17.10.2020 09:01


World Languages, 17.10.2020 09:01

Mathematics, 17.10.2020 09:01


Biology, 17.10.2020 09:01



Mathematics, 17.10.2020 09:01



