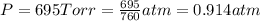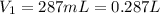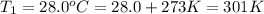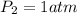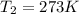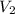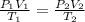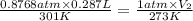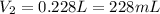Chemistry, 27.03.2020 01:04 slycooper99
A cylinder of compressed gas rolls off a boat and falls to the bottom of a lake. Eventually it rusts and the gas bubbles to the surface. A chemist collects a sample of the gas with the idea of trying to identify the gas. The wet gas collected occupies a volume of 287 mL at a pressure of 695 torr and temperature of 28.0oC. The vapor pressure of water at 28.0oC is 0.0372 atm. 1. Calculate the volume (L) that the gas occupies after it is dried (the water vapor removed) and stored at STP.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
A cylinder of compressed gas rolls off a boat and falls to the bottom of a lake. Eventually it rusts...
Questions



Mathematics, 21.01.2021 07:30

History, 21.01.2021 07:30


Mathematics, 21.01.2021 07:30


Mathematics, 21.01.2021 07:30

Mathematics, 21.01.2021 07:30


Mathematics, 21.01.2021 07:30



Social Studies, 21.01.2021 07:40

Mathematics, 21.01.2021 07:40




Mathematics, 21.01.2021 07:40

Mathematics, 21.01.2021 07:40