
Chemistry, 27.03.2020 01:00 braydenmcd02
Calculate the pKa of acetic acid and the pKb of methylamine (CH3NH2). Acetic acid’s Ka is 1.8 x 10-5. Methylamine’s Ka is 4.2 x 10-4.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1
You know the right answer?
Calculate the pKa of acetic acid and the pKb of methylamine (CH3NH2). Acetic acid’s Ka is 1.8 x 10-5...
Questions



Chemistry, 19.11.2019 02:31



Mathematics, 19.11.2019 02:31

Mathematics, 19.11.2019 02:31






Mathematics, 19.11.2019 02:31


History, 19.11.2019 02:31


Biology, 19.11.2019 02:31



Social Studies, 19.11.2019 02:31

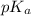 of acetic acid is 4.7.
of acetic acid is 4.7.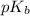 of methylamine is 3.4.
of methylamine is 3.4.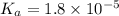
![pK_a=-\log[K_a]](/tpl/images/0566/4951/78bbf.png)
![pK_a=-\log[1.8\times 10^{-5}]=4.7](/tpl/images/0566/4951/353fe.png)
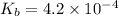
![pK_b=-\log[K_b]](/tpl/images/0566/4951/b72fe.png)
![pK_b=-\log[4.2\times 10^{-4}]=3.4](/tpl/images/0566/4951/6fbec.png)


