
Chemistry, 27.03.2020 01:29 mcckenziee
When the oxide of generic metal M is heated at 25.0 ∘ C , a negligible amount of M is produced. MO 2 ( s ) − ⇀ ↽ − M ( s ) + O 2 ( g ) Δ G ∘ = 291.0 kJ mol When this reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. What is the chemical equation of this coupled process? Show that the reaction is in equilibrium. Include physical states and represent graphite as C ( s ) .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

You know the right answer?
When the oxide of generic metal M is heated at 25.0 ∘ C , a negligible amount of M is produced. MO 2...
Questions

Mathematics, 03.10.2019 05:00

History, 03.10.2019 05:00


Mathematics, 03.10.2019 05:00


Chemistry, 03.10.2019 05:00

Mathematics, 03.10.2019 05:00


Spanish, 03.10.2019 05:00



Geography, 03.10.2019 05:00

Mathematics, 03.10.2019 05:00

Mathematics, 03.10.2019 05:00

Biology, 03.10.2019 05:00



English, 03.10.2019 05:00


Chemistry, 03.10.2019 05:00

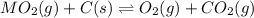
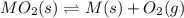 .....[1]
.....[1]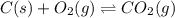 ..[2]
..[2]

