
Chemistry, 27.03.2020 01:50 marsewilliams
Suppose the reaction system below has already reached equilibrium. Predict the effect that each of the following changes will have on the equilibrium position. Tell whether the equilibrium will shift to the right, will shift to the left, or will not be affected.
UO2(s) + 4 HF(g) ↔ UF4(g) + 2 H2O(g)
1. Water vapor is removed
2. UF4(g) is added
3. The reaction is done in a glass reaction vessel. HF(g) attacks and reacts with the glass
4. If the reaction is endothermic, and you want to make the equilibrium shift to the right to maximize the products, would you heat or cool the reaction vessel?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2


Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
Suppose the reaction system below has already reached equilibrium. Predict the effect that each of t...
Questions

Mathematics, 02.08.2019 22:00






Mathematics, 02.08.2019 22:00

English, 02.08.2019 22:00




Physics, 02.08.2019 22:00

Mathematics, 02.08.2019 22:00

History, 02.08.2019 22:00




Mathematics, 02.08.2019 22:00


English, 02.08.2019 22:00

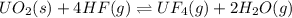
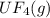 is added
is added

