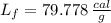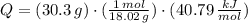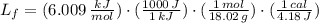Chemistry, 27.03.2020 04:29 dontcareanyonemo
The molar enthalpy of vaporization of water is 40.79 kJ/mol, and the molar enthalpy of fusion of ice is 6.009 kJ/mol. The molar mass of water is 18.02 g/mol. a. How much energy is absorbed when 30.3 g of liquid water boils? b. An energy unit often encountered is the calorie (4.18 J = 1 calorie). Determine the molar enthalpy of fusion of ice in calories per gram.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
The molar enthalpy of vaporization of water is 40.79 kJ/mol, and the molar enthalpy of fusion of ice...
Questions


Social Studies, 17.05.2021 06:30


Mathematics, 17.05.2021 06:30


Mathematics, 17.05.2021 06:30


Mathematics, 17.05.2021 06:30



Mathematics, 17.05.2021 06:30



Mathematics, 17.05.2021 06:30

Spanish, 17.05.2021 06:30

Chemistry, 17.05.2021 06:30


History, 17.05.2021 06:30


Mathematics, 17.05.2021 06:40

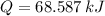 , b)
, b)