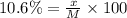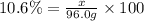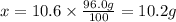Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3


You know the right answer?
What mass of KNO3 is present in 96.0 g of a solution that is 10.6% KNO3 by mass?...
Questions








Computers and Technology, 27.11.2019 23:31










Chemistry, 27.11.2019 23:31