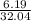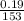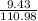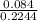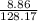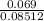Chemistry, 27.03.2020 19:31 carlosleblanc26
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methanol (CH3OH) in 1.50 × 102 mL of solution:
mol/L
(b) 9.43 g of calcium chloride (CaCl2) in 2.20 × 102 mL of solution:
mol/L
(c) 8.86 g of naphthalene (C10H8) in 85.2 mL of benzene solution:
mol/L

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1


Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methano...
(a) 6.19 g of methano...
Questions

Social Studies, 26.11.2020 08:20

Social Studies, 26.11.2020 08:20


Social Studies, 26.11.2020 08:20

Mathematics, 26.11.2020 08:20



History, 26.11.2020 08:20

Mathematics, 26.11.2020 08:20

Social Studies, 26.11.2020 08:20

Advanced Placement (AP), 26.11.2020 08:20

Mathematics, 26.11.2020 08:20

Mathematics, 26.11.2020 08:20

Mathematics, 26.11.2020 08:20


Mathematics, 26.11.2020 08:20

English, 26.11.2020 08:20

History, 26.11.2020 08:20


Mathematics, 26.11.2020 08:20

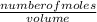 equation 1
equation 1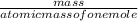 equation 2
equation 2