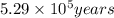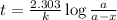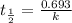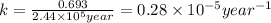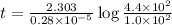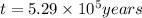Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If there are 4.4 x 102 g of the isotope in a small atomic bomb, how long will it take (in yr) for the substance to decay to 1.0 x 102 g, too small an amount for an effective bomb? This radioactive decay follows first order kinetics.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If ther...
Questions

English, 25.01.2022 22:10


Mathematics, 25.01.2022 22:20



Business, 25.01.2022 22:20

Mathematics, 25.01.2022 22:20

History, 25.01.2022 22:20

Mathematics, 25.01.2022 22:20

Mathematics, 25.01.2022 22:30

English, 25.01.2022 22:30

Physics, 25.01.2022 22:30

Computers and Technology, 25.01.2022 22:30




Chemistry, 25.01.2022 22:30

Mathematics, 25.01.2022 22:30

Mathematics, 25.01.2022 22:30

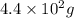 to
to  is
is