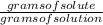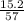Chemistry, 27.03.2020 19:50 shradhwaip2426
Boiling point of a solution formed when 15.2 grams of CaCl2 dissolves in 57.0 g of water. kB= 0.512 c/m.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 23.06.2019 11:20
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2

Chemistry, 23.06.2019 14:40
Uuestons niuthe no. of millimoles of hcl required to neutralize 10 ml of 0.2 m na2co3 is(a) 2.0 m mole(b) 4.0 m mole(c) 0.2 m mole(d) 0.4 m mole
Answers: 1

Chemistry, 23.06.2019 14:50
Use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 +473 kj +206 kj -392 kj -91 kj -486 kj
Answers: 1
You know the right answer?
Boiling point of a solution formed when 15.2 grams of CaCl2 dissolves in 57.0 g of water. kB= 0.512...
Questions


Physics, 26.05.2021 09:10

Mathematics, 26.05.2021 09:10

Mathematics, 26.05.2021 09:10

Mathematics, 26.05.2021 09:10

Mathematics, 26.05.2021 09:10

Mathematics, 26.05.2021 09:10


Mathematics, 26.05.2021 09:10

Mathematics, 26.05.2021 09:10

Mathematics, 26.05.2021 09:10


Biology, 26.05.2021 09:10

Biology, 26.05.2021 09:10

Mathematics, 26.05.2021 09:10


Mathematics, 26.05.2021 09:10