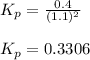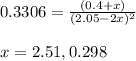Chemistry, 27.03.2020 21:04 ayoismeisjjjjuan
Nitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOx") that are of interest to atmospheric chemistry. It can react with itself to form another form of NOx, dinitrogen tetroxide. A chemical engineer studying this reaction fills a 5.0L flask at 10.°C with 1.9atm of nitrogen dioxide gas. He then raises the temperature considerably, and when the mixture has come to equilibrium determines that it contains 1.1atm of nitrogen dioxide gas. The engineer then adds another 0.95 atm of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the pressure of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

You know the right answer?
Nitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOx") that are of...
Questions



Mathematics, 12.10.2020 14:01


French, 12.10.2020 14:01

History, 12.10.2020 14:01


History, 12.10.2020 14:01

Spanish, 12.10.2020 14:01

Social Studies, 12.10.2020 14:01









Mathematics, 12.10.2020 14:01

Mathematics, 12.10.2020 14:01

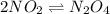
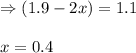
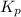 for above equation follows:
for above equation follows: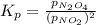 ..........(1)
..........(1)