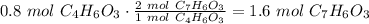Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

You know the right answer?
Salicylic acid (c7h6o3) reacts with acetic anhydride (c4h6o3) to form acetylsalicylic acid (c9h8o4)....
Questions

Computers and Technology, 22.12.2020 23:30

English, 22.12.2020 23:30

Mathematics, 22.12.2020 23:30






Chemistry, 22.12.2020 23:30


Biology, 22.12.2020 23:30

Mathematics, 22.12.2020 23:30

Computers and Technology, 22.12.2020 23:30



Biology, 22.12.2020 23:30


History, 22.12.2020 23:30


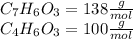
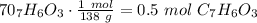
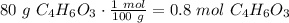
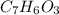 are necessary to react with 1 mol of
are necessary to react with 1 mol of 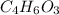 . Following this ratio:
. Following this ratio: