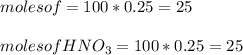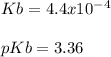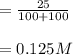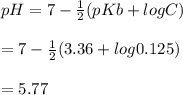A 100 mL sample of 0.25 M CH3NH2(aq) is titrated with a 100 mL of 0.25 M HNO3(aq). Select ALL main components (greater than 0.001 moles, besides H2O) that would be present in the solution after adding HNO3. Kb of CH3NH2 is 4.4 LaTeX: \times×10−4.
OH−
CH3NH2
NO3−
CH3NH3+

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
A 100 mL sample of 0.25 M CH3NH2(aq) is titrated with a 100 mL of 0.25 M HNO3(aq). Select ALL main c...
Questions


Chemistry, 08.03.2021 21:20

Arts, 08.03.2021 21:20

English, 08.03.2021 21:20

History, 08.03.2021 21:20

History, 08.03.2021 21:20



Spanish, 08.03.2021 21:20

Mathematics, 08.03.2021 21:20

Geography, 08.03.2021 21:20

Spanish, 08.03.2021 21:20

Geography, 08.03.2021 21:20

Chemistry, 08.03.2021 21:20

English, 08.03.2021 21:20

Mathematics, 08.03.2021 21:20

Mathematics, 08.03.2021 21:20



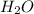 ) that would be present in the solution after adding
) that would be present in the solution after adding 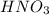 ."
." 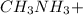 would be present in the solution after adding
would be present in the solution after adding 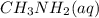 is titrated with a 100 mL of 0.25 M
is titrated with a 100 mL of 0.25 M 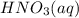 .
.