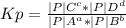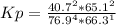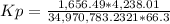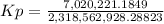Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2(g) → 2 Cl2(g) + 2 H2O(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen chloride, oxygen, chlorine, and water has the following composition:
COMPOUND Pressure at equilibrium
HCl 76.9 atm
O2 66.3 atm
Cl2 40.7 atm
H2O 65.1 atm
Calculate the value of the equilibrium constant Kp for this reaction. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2...
4 HCl(g) + O2...
Questions



Mathematics, 06.04.2020 02:13




Mathematics, 06.04.2020 02:15

Mathematics, 06.04.2020 02:15

History, 06.04.2020 02:15

English, 06.04.2020 02:16

Mathematics, 06.04.2020 02:16



Biology, 06.04.2020 02:16



Mathematics, 06.04.2020 02:16

Mathematics, 06.04.2020 02:16

Social Studies, 06.04.2020 02:17

History, 06.04.2020 02:17