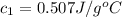Chemistry, 28.03.2020 17:59 meiyrarodriguez
A 24.87 gram sample of a metal at 104.0ºC was added to 76.12 grams of water at 25.2ºC in a perfectly insulated calorimeter. The final temperature of the metal-water mixture was 28.2ºC. Calculate the specific heat capacity of the metal using the data. Type your work and answer below. Make sure to include a unit on the final answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3

You know the right answer?
A 24.87 gram sample of a metal at 104.0ºC was added to 76.12 grams of water at 25.2ºC in a perfectly...
Questions

History, 01.09.2019 09:00

World Languages, 01.09.2019 09:00



History, 01.09.2019 09:00

Computers and Technology, 01.09.2019 09:00

History, 01.09.2019 09:00



Mathematics, 01.09.2019 09:00

Mathematics, 01.09.2019 09:00


Mathematics, 01.09.2019 09:00

Mathematics, 01.09.2019 09:00

English, 01.09.2019 09:00

Mathematics, 01.09.2019 09:00

Biology, 01.09.2019 09:00

Mathematics, 01.09.2019 09:00


Mathematics, 01.09.2019 09:00

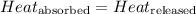
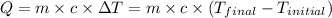
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0568/9675/09236.png) ......(1)
......(1)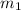 = mass of metal = 24.87 g
= mass of metal = 24.87 g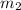 = mass of water = 76.12 g
= mass of water = 76.12 g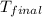 = final temperature = 28.2°C
= final temperature = 28.2°C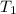 = initial temperature of metal = 104.0°C
= initial temperature of metal = 104.0°C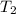 = initial temperature of water = 25.2°C
= initial temperature of water = 25.2°C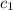 = specific heat of metal = ?
= specific heat of metal = ?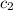 = specific heat of water = 4.186 J/g°C
= specific heat of water = 4.186 J/g°C![24.87\times c_1\times (28.2-104)=-[76.12\times 4.186\times (28.2-25.2)]](/tpl/images/0568/9675/a6f4b.png)