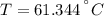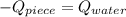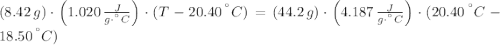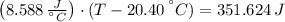Chemistry, 28.03.2020 18:38 veroushkarose7326
An 8.42 g piece of metal with a specific heat of 1.020 J g-1 k-1 wasn't heated to an unknown temperature. The metal was then placed in 44.2 g of water with an initial temperature of 18.50 C. If the final temperature of the water was 20.40 C, what temperature was the metal initially heated to (in C).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
An 8.42 g piece of metal with a specific heat of 1.020 J g-1 k-1 wasn't heated to an unknown tempera...
Questions


Spanish, 12.10.2019 11:20



History, 12.10.2019 11:20

Mathematics, 12.10.2019 11:20

History, 12.10.2019 11:20

Mathematics, 12.10.2019 11:20

Biology, 12.10.2019 11:20

Geography, 12.10.2019 11:20




Social Studies, 12.10.2019 11:20

Biology, 12.10.2019 11:20

English, 12.10.2019 11:20


History, 12.10.2019 11:20


Mathematics, 12.10.2019 11:30