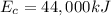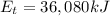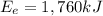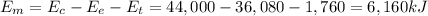Chemistry, 28.03.2020 21:15 daniellaZemira
Gasoline containing a total of 44,000 kJ of energy was burned in a car
engine. The table below shows some information related to the energy
output of the car from burning the gasoline. How much potential energy
from the gasoline was converted to mechanical energy? Energy Output
in (kJ) Thermal = 36,080 Electrical = 1,760 Mechanical = ?? *
6,160 kJ
14,960 kJ
0
20,240 kJ
44,000 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
Gasoline containing a total of 44,000 kJ of energy was burned in a car
engine. The table below...
engine. The table below...
Questions




Mathematics, 26.09.2021 17:50

Medicine, 26.09.2021 17:50

Mathematics, 26.09.2021 17:50

Social Studies, 26.09.2021 17:50



History, 26.09.2021 17:50


Mathematics, 26.09.2021 17:50

Mathematics, 26.09.2021 17:50



Mathematics, 26.09.2021 17:50


Mathematics, 26.09.2021 17:50


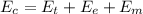
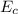 is the chemical energy contained in the gasoline (the energy in input)
is the chemical energy contained in the gasoline (the energy in input)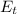 is the thermal energy in output
is the thermal energy in output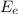 is the electrical energy in output
is the electrical energy in output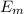 is the mechanical energy in output
is the mechanical energy in output