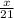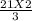Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
...

Chemistry, 28.03.2020 22:17 NetherisIsTheQueen
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
3H2(g)+N2(g)→2NH3(g)
How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
You know the right answer?
Questions



English, 10.11.2020 22:00




Arts, 10.11.2020 22:00


Mathematics, 10.11.2020 22:00

English, 10.11.2020 22:00







Mathematics, 10.11.2020 22:00


Mathematics, 10.11.2020 22:00

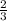 =
=