
Chemistry, 29.03.2020 01:30 madiiiiiii69
If a 210.5g sample of phenol (C6H5OH) is isolated in a laboratory how many
moles of hydrogen are within phenol. (C6H5OH=94.11g/mol]
0.372 mol H
13.4 mol H.
11.2 mol H
2.23 mol H

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2


Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
If a 210.5g sample of phenol (C6H5OH) is isolated in a laboratory how many
moles of hydrogen a...
moles of hydrogen a...
Questions

English, 19.10.2019 17:30



Mathematics, 19.10.2019 17:30


Business, 19.10.2019 17:30

English, 19.10.2019 17:30


Mathematics, 19.10.2019 17:30

Mathematics, 19.10.2019 17:30

Physics, 19.10.2019 17:30





History, 19.10.2019 17:30


English, 19.10.2019 17:30

Computers and Technology, 19.10.2019 17:30

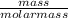
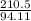 = 2.24mol
= 2.24mol

