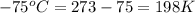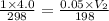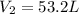Chemistry, 29.03.2020 17:40 ashcookie27
A 4.0L weather balloon is launched from sea level where the temperature is 25°C. It rises to an altitude where the pressure is 0.05atm and the temperature is -75°C. If the balloon has the capacity to expand to a 50 liter volume before bursting, will the balloon survive?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

You know the right answer?
A 4.0L weather balloon is launched from sea level where the temperature is 25°C. It rises to an alti...
Questions

Mathematics, 25.04.2020 05:46

Mathematics, 25.04.2020 05:46

Mathematics, 25.04.2020 05:46


History, 25.04.2020 05:46

Mathematics, 25.04.2020 05:46

Physics, 25.04.2020 05:46



Mathematics, 25.04.2020 05:46

Mathematics, 25.04.2020 05:46

Mathematics, 25.04.2020 05:46

Mathematics, 25.04.2020 05:46

Mathematics, 25.04.2020 05:46



Mathematics, 25.04.2020 05:46

Geography, 25.04.2020 05:46

Mathematics, 25.04.2020 05:46

English, 25.04.2020 05:46

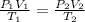
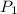 = initial pressure of gas = 1 atm
= initial pressure of gas = 1 atm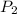 = final pressure of gas = 0.05 atm
= final pressure of gas = 0.05 atm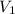 = initial volume of gas = 4.0 L
= initial volume of gas = 4.0 L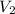 = final volume of gas = ?
= final volume of gas = ?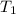 = initial temperature of gas =
= initial temperature of gas = 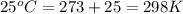
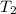 = final temperature of gas =
= final temperature of gas =