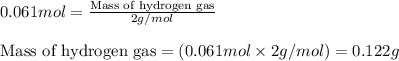Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
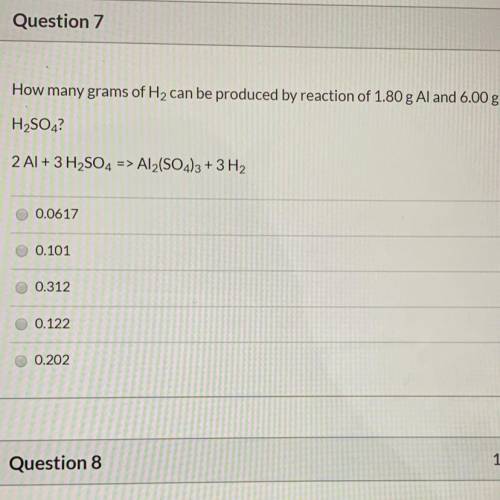
How many grams of H2 can be produced by reaction of 1.80 g Al and 6.00 g
H2SO4?
2 Al + 3...
H2SO4?
2 Al + 3...
Questions

History, 14.04.2021 06:20



French, 14.04.2021 06:20

Mathematics, 14.04.2021 06:20

Mathematics, 14.04.2021 06:20




Mathematics, 14.04.2021 06:20


History, 14.04.2021 06:20

Mathematics, 14.04.2021 06:20



Mathematics, 14.04.2021 06:20



Mathematics, 14.04.2021 06:20

History, 14.04.2021 06:20

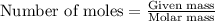 .....(1)
.....(1)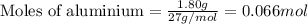
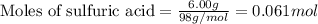
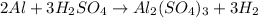
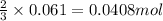 of aluminium
of aluminium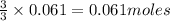 of hydrogen gas
of hydrogen gas