
Chemistry, 29.03.2020 22:17 alligatorsocks
25.0 g of a substance requires 117.4 Joules of energy to raise its temperature from 75.0 to 87.2 oC. What is the specific heat of the substance?
.054 J/g°C
.063 J/g°C
2.60 J/g°C
.385 J/g°C

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
25.0 g of a substance requires 117.4 Joules of energy to raise its temperature from 75.0 to 87.2 oC....
Questions




Computers and Technology, 03.10.2019 00:30


Mathematics, 03.10.2019 00:30

Computers and Technology, 03.10.2019 00:30












Mathematics, 03.10.2019 00:30

Biology, 03.10.2019 00:30

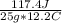 = 0.385 J/g°C
= 0.385 J/g°C

