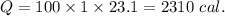Chemistry, 29.03.2020 22:37 Candieboo6939
In lab, a student burns 0.550 grams of Ritz crackers. The burning causes 100.0 grams of water to rise from 24.0°C go 47.1 °C.
Calculate the number of calories absorbed by the WATER as the Ritz crackers burn. (Use the specific heat of water in terms of calories: 1.0 cal/ g °C) ** Remember you are calculating the heat gained by the water, NOT the heat lost by the Ritz cracker - use the correct mass!!
12.7 cal
53.2 cal
2310 cal
9.67 x 105 cal

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1


Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1
You know the right answer?
In lab, a student burns 0.550 grams of Ritz crackers. The burning causes 100.0 grams of water to ris...
Questions

English, 04.02.2020 01:48



Mathematics, 04.02.2020 01:48

Social Studies, 04.02.2020 01:48

Mathematics, 04.02.2020 01:48

Chemistry, 04.02.2020 01:48



History, 04.02.2020 01:48



History, 04.02.2020 01:48

Geography, 04.02.2020 01:48

History, 04.02.2020 01:48



History, 04.02.2020 01:48

Mathematics, 04.02.2020 01:48

Mathematics, 04.02.2020 01:48