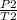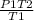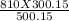Chemistry, 30.03.2020 01:35 gatorlove00
A gas has a pressure of 810 kPa at 227°C. What will its pressure be at 27°C?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2
You know the right answer?
A gas has a pressure of 810 kPa at 227°C. What will its pressure be at 27°C?...
Questions

Mathematics, 12.03.2021 19:30



Chemistry, 12.03.2021 19:30


Mathematics, 12.03.2021 19:30


Mathematics, 12.03.2021 19:30

Mathematics, 12.03.2021 19:30

Mathematics, 12.03.2021 19:30



Mathematics, 12.03.2021 19:30

Mathematics, 12.03.2021 19:30

Chemistry, 12.03.2021 19:30


Mathematics, 12.03.2021 19:30

Mathematics, 12.03.2021 19:30


Mathematics, 12.03.2021 19:30

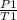 =
=