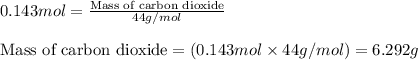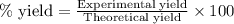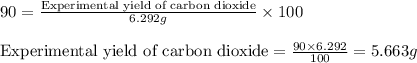Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
Consider the balanced equation for the following reaction:
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(...
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(...
Questions


English, 29.08.2019 07:00

English, 29.08.2019 07:00

Mathematics, 29.08.2019 07:00


Mathematics, 29.08.2019 07:00

Mathematics, 29.08.2019 07:00








Geography, 29.08.2019 07:00





Health, 29.08.2019 07:00

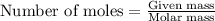 .....(1)
.....(1)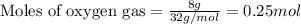
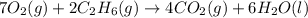
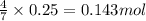 of carbon dioxide
of carbon dioxide