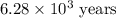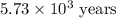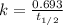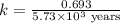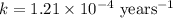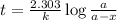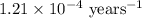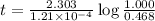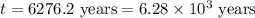Chemistry, 30.03.2020 15:41 SMURFETTE86
The half-life for the radioactive decay of carbon-14 to nitrogen-14 is 5.73 x 10^3 years. Suppose nuclear chemical analysis shows that there is 0.523mmol of nitrogen-14 for every 1.000 mmol of carbon-14 in a certain sample of rock.
Calculate the age of the rock. Round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
The half-life for the radioactive decay of carbon-14 to nitrogen-14 is 5.73 x 10^3 years. Suppose nu...
Questions




Mathematics, 03.02.2020 23:49


Biology, 03.02.2020 23:49


Computers and Technology, 03.02.2020 23:49







English, 03.02.2020 23:49




Mathematics, 03.02.2020 23:49