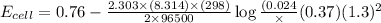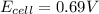In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+ half-cell and an H2/H+ half-cell under the following conditions: [Zn2+ ] = 0.024 M [H+ ]= 1.3 M partial pressure of H2 = 0.37 atm. Calculate Ecell at 298 K (enter to 3 decimal places). Zn2+ (aq) + 2eLaTeX: -− LaTeX: \longrightarrow⟶ Zn(s) E° = LaTeX: -−0.76 V 2H+ (aq) + 2eLaTeX: -−LaTeX: \longrightarrow⟶ H2(g) E° = 0.00 V

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
True or false, the three major scales used to measure earthquakes are mercalli scale, richter scale and magnitude scale
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+...
Questions

Mathematics, 12.12.2021 09:20



Physics, 12.12.2021 09:30

History, 12.12.2021 09:30

Mathematics, 12.12.2021 09:30

Mathematics, 12.12.2021 09:30


Health, 12.12.2021 09:30



Business, 12.12.2021 09:30

Computers and Technology, 12.12.2021 09:30




Business, 12.12.2021 09:30

Computers and Technology, 12.12.2021 09:30

History, 12.12.2021 09:30

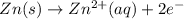
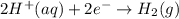
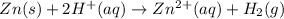
![E^o_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0570/4548/c67da.png)
![E^o_{[H^+/H_2]}=0.00V](/tpl/images/0570/4548/4b48a.png)
![E^o=E^o_{[cathode]}-E^o_{[anode]}](/tpl/images/0570/4548/51f3e.png)
![E^o=E^o_{[H^+/H_2]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0570/4548/91ffc.png)
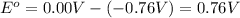
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]\times (p_{H_2})}{[H^+]^2}](/tpl/images/0570/4548/307e3.png)
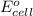 = electrode potential of the cell = ?
= electrode potential of the cell = ?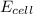 = emf of the cell = 0.76 V
= emf of the cell = 0.76 V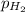 = 0.37 atm
= 0.37 atm![[Zn^{2+}]](/tpl/images/0570/4548/9c01a.png) = 0.024 M
= 0.024 M![[H^{+}]](/tpl/images/0570/4548/85507.png) = 1.3 M
= 1.3 M