
Chemistry, 30.03.2020 17:18 yesman1288
Consider: CO(g) + Cl2 (g) ⇌ COCl2 (g) Kc = 1.2×103 at 395 °C. If the equilibrium concentrations of Cl2 and COCl2 are the same at 395 °C, find the equilibrium concentration of CO in the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:02
What is the partial pressure of ethane, pethane, in the flask?
Answers: 1

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1


You know the right answer?
Consider: CO(g) + Cl2 (g) ⇌ COCl2 (g) Kc = 1.2×103 at 395 °C. If the equilibrium concentrations of C...
Questions



Mathematics, 17.12.2020 21:30

Computers and Technology, 17.12.2020 21:30

Mathematics, 17.12.2020 21:30

Biology, 17.12.2020 21:30


Mathematics, 17.12.2020 21:30

Social Studies, 17.12.2020 21:30




Mathematics, 17.12.2020 21:30




Mathematics, 17.12.2020 21:30

Computers and Technology, 17.12.2020 21:30


English, 17.12.2020 21:30

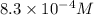
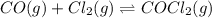
![K_c=\frac{[COCl_2]}{[CO][Cl_2]}](/tpl/images/0570/4835/36d91.png)
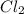 at equilibrium = Concentration of
at equilibrium = Concentration of 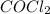
![K_c=\frac{[Cl_2]}{[CO][Cl_2]}](/tpl/images/0570/4835/18881.png)
![K_c=\frac{1}{[CO]}](/tpl/images/0570/4835/6d26f.png)
![1.2\times 10^3=\frac{1}{[CO]}](/tpl/images/0570/4835/d1c27.png)
![[CO]=8.3\times 10^{-4}M](/tpl/images/0570/4835/cb9b9.png)


