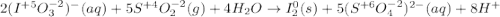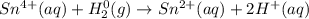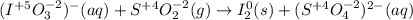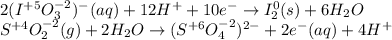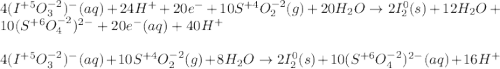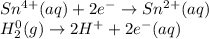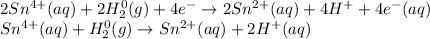Chemistry, 30.03.2020 17:20 irvinanderson
Balance each redox reaction occurring in acidic aqueous solution. Use the half-reaction method. Part A IO3−(aq)+SO2(g)→I2(s)+SO42−(aq)IO3− (aq)+SO2(g)→I2(s)+SO42−(aq) Express your answer as a chemical equation. Identify all of the phases in your answer. nothing SubmitRequest Answer Part B Sn4+(aq)+H2(g)→Sn2+(aq)+H+(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1

Chemistry, 23.06.2019 09:30
What lessons does the history and study of the periodic table offer to other fields of science, and the pursuit science more generally
Answers: 3

Chemistry, 23.06.2019 11:50
Charles's law describes the relationship of the volume and temperature of gas at a constant mass and pressure. according to this law, what would happen to the temperature of the gas if its volume decreased from 1.0 l to 0.50 l?
Answers: 3
You know the right answer?
Balance each redox reaction occurring in acidic aqueous solution. Use the half-reaction method. Part...
Questions




Biology, 11.02.2020 01:33



Mathematics, 11.02.2020 01:33

Mathematics, 11.02.2020 01:33


Mathematics, 11.02.2020 01:33




Physics, 11.02.2020 01:33