Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1

Chemistry, 23.06.2019 10:30
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1
You know the right answer?
In a liquid mixture of chloroform (C) and acetone (A) with chloroform mole fraction xC = 0.4693, the...
Questions

History, 21.08.2020 19:01

Computers and Technology, 21.08.2020 19:01

History, 21.08.2020 19:01







History, 21.08.2020 19:01




Social Studies, 21.08.2020 19:01





Computers and Technology, 21.08.2020 19:01

English, 21.08.2020 19:01

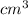
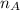
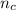
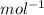
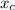 ) × 58.08 + n
) × 58.08 + n 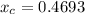
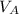 +
+