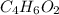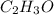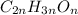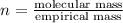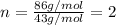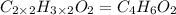Chemistry, 30.03.2020 17:30 legend101xD
Severus Snape explains that if you have the molar mass of a compound and the empirical formula of a compound you can determine the molecular formula of a compound. Suppose you have a compound which has an empirical formula of C2H3O and molar mass of 86 g/mol. What is the compounds molecular formula

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
Severus Snape explains that if you have the molar mass of a compound and the empirical formula of a...
Questions

English, 28.06.2019 06:30

Mathematics, 28.06.2019 06:30


Mathematics, 28.06.2019 06:30

Mathematics, 28.06.2019 06:30

Business, 28.06.2019 06:30



Chemistry, 28.06.2019 06:30

History, 28.06.2019 06:30

Mathematics, 28.06.2019 06:30


Mathematics, 28.06.2019 06:30



Arts, 28.06.2019 06:30