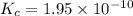Chemistry, 30.03.2020 17:28 natalie2sheffield
G Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) + 3O2(g) When this reaction was run at room temperature, the following equilbrium concentrations were measured: [O2] = 0.0500 M; [KCl] = 0.00250 M; [KClO3] = 2.00 M What is the equilibrium constant for this reaction?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
G Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) +...
Questions






Health, 17.03.2020 20:07

Geography, 17.03.2020 20:07




Biology, 17.03.2020 20:07

Mathematics, 17.03.2020 20:07



Computers and Technology, 17.03.2020 20:07



Biology, 17.03.2020 20:07


Biology, 17.03.2020 20:07

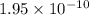 is the equilibrium constant for this reaction.
is the equilibrium constant for this reaction.![[O_2] = 0.0500 M](/tpl/images/0570/5147/94139.png)
![[KCl] = 0.00250 M](/tpl/images/0570/5147/3a302.png)
![[KClO_3] = 2.00 M](/tpl/images/0570/5147/90bf6.png)
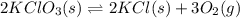
![K_c=\frac{[KCl]^2[O_2]^3}{[KClO_3]^2}](/tpl/images/0570/5147/bd160.png)
![=\frac{[0.00250 M]^2[0.0500 M]^3}{[2.00 M]^2}](/tpl/images/0570/5147/c5974.png)