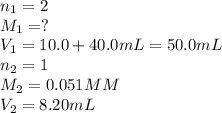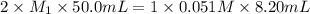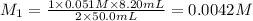Chemistry, 30.03.2020 17:44 allisonorlov
In the titration of wine to determine the acid concentration, 10.0 mL of wine was placed in a beaker and diluted with 40.0 mL of water. 8.20 mL of 0.051 M NaOH was required to reach the endpoint. Remembering that the acid in wine is tartaric acid, a diprotic acid, what is the molarity (M/L) of tartaric acid in this sample of wine

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3


Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
In the titration of wine to determine the acid concentration, 10.0 mL of wine was placed in a beaker...
Questions



Mathematics, 19.11.2020 18:00

Mathematics, 19.11.2020 18:00

Mathematics, 19.11.2020 18:00

English, 19.11.2020 18:00

Mathematics, 19.11.2020 18:00

Mathematics, 19.11.2020 18:00



Social Studies, 19.11.2020 18:00


Mathematics, 19.11.2020 18:00

History, 19.11.2020 18:00



Mathematics, 19.11.2020 18:00


Social Studies, 19.11.2020 18:00

Social Studies, 19.11.2020 18:00

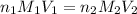
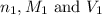 are the n-factor, molarity and volume of acid which is tartaric acid
are the n-factor, molarity and volume of acid which is tartaric acid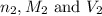 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.