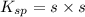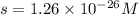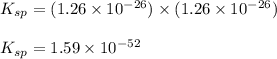Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
At 25 oC the solubility of mercury(II) sulfide is 1.26 x 10-26 mol/L. Calculate the value of Ksp at...
Questions


Mathematics, 07.11.2019 03:31

Arts, 07.11.2019 03:31



Mathematics, 07.11.2019 03:31



Mathematics, 07.11.2019 03:31


Mathematics, 07.11.2019 03:31




Biology, 07.11.2019 03:31

Geography, 07.11.2019 03:31


Mathematics, 07.11.2019 03:31


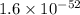
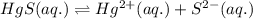
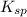 for above equation follows:
for above equation follows: