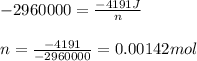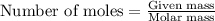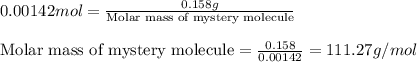Chemistry, 30.03.2020 18:52 Herbie3070
0.158 g of a mystery molecule is placed into a bomb calorimeter that has a heat capacity of 1650 J/C. After the sample is combusted the temperature of the calorimeter increased by 2.54 C. Determine the molar mass of the mystery molecule if the enthalpy of combustion for one mole is 2960 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
0.158 g of a mystery molecule is placed into a bomb calorimeter that has a heat capacity of 1650 J/C...
Questions

English, 16.10.2020 03:01


Chemistry, 16.10.2020 03:01



English, 16.10.2020 03:01



Chemistry, 16.10.2020 03:01






Chemistry, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01

English, 16.10.2020 03:01

Biology, 16.10.2020 03:01

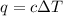
 = change in temperature =
= change in temperature = 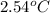
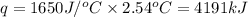
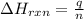
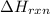 = enthalpy change of the reaction = -2960 kJ/mol = -2960000 J/mol (Conversion factor = 1 kJ = 1000 J)
= enthalpy change of the reaction = -2960 kJ/mol = -2960000 J/mol (Conversion factor = 1 kJ = 1000 J)