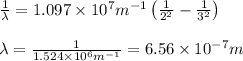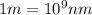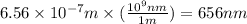Chemistry, 30.03.2020 18:45 rileyeddins1010
Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the transition from the energy level n = 3 n=3 to the level n = 2 .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydroge...
Questions

History, 29.07.2019 14:30




History, 29.07.2019 14:30







Mathematics, 29.07.2019 14:30

History, 29.07.2019 14:30


Mathematics, 29.07.2019 14:30

Mathematics, 29.07.2019 14:30

History, 29.07.2019 14:30



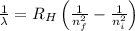
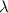 = Wavelength of radiation
= Wavelength of radiation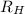 = Rydberg's Constant =
= Rydberg's Constant = 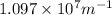
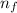 = Final energy level = 2
= Final energy level = 2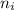 = Initial energy level = 3
= Initial energy level = 3