
A sealed isothermal container initially contains 4 moles of methanol gas, CH3OH. The following reversible reaction occurs: CH3OH (g) D 2 H2 (g) +CO (g) At equilibrium, there were 3 moles of CH3OH in the container. What is the total number of moles of gas present in the container at equilibrium?

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2

Chemistry, 23.06.2019 11:00
The standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) → 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. the standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. 2.36 2.24 2.21 2.51 2.04
Answers: 2

You know the right answer?
A sealed isothermal container initially contains 4 moles of methanol gas, CH3OH. The following rever...
Questions

Mathematics, 28.12.2020 19:30

Mathematics, 28.12.2020 19:30

Mathematics, 28.12.2020 19:30

Mathematics, 28.12.2020 19:30




Computers and Technology, 28.12.2020 19:30

Social Studies, 28.12.2020 19:30

Mathematics, 28.12.2020 19:30


Mathematics, 28.12.2020 19:30

Computers and Technology, 28.12.2020 19:30



Mathematics, 28.12.2020 19:30


Mathematics, 28.12.2020 19:30


Mathematics, 28.12.2020 19:30


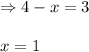
![[n_{CH_3OH}+n_{H_2}+n_{CO}]=[3+2+1]=6mol](/tpl/images/0570/7796/f27cd.png)


