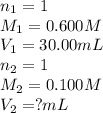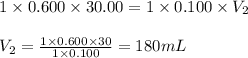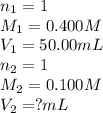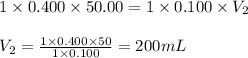Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Determine the volume at the equivalence point if a 0.100 M NaOH(aq) solution is used to titrate the...
Questions




Mathematics, 22.12.2020 09:00



Mathematics, 22.12.2020 09:00





Mathematics, 22.12.2020 09:00

Mathematics, 22.12.2020 09:00


Physics, 22.12.2020 09:00



History, 22.12.2020 09:00

English, 22.12.2020 09:00

Mathematics, 22.12.2020 09:00

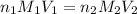
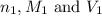 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid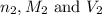 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base