
Chemistry, 30.03.2020 19:07 turtlesage21
G For the reaction N2(g) + O2(g) --> 2 NO(g), the equilibrium constant, K=1.0x10-5 Which direction will the reaction move, and why, if the reactants and products have the following concentrations: [N2]=0.015 M [O2]=0.25 M [NO]=0.010

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
G For the reaction N2(g) + O2(g) --> 2 NO(g), the equilibrium constant, K=1.0x10-5 Which directio...
Questions



History, 10.10.2019 07:30

Mathematics, 10.10.2019 07:30

Mathematics, 10.10.2019 07:30




Business, 10.10.2019 07:30

Mathematics, 10.10.2019 07:30








Biology, 10.10.2019 07:30


Mathematics, 10.10.2019 07:30

![\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0570/7732/10229.png) where the terms in the large bracket denote the concentrations of respective reacting species.
where the terms in the large bracket denote the concentrations of respective reacting species.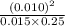 =
= 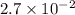
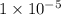 , we see that the Q is larger than that of K.
, we see that the Q is larger than that of K.

