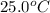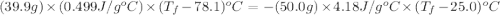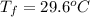A hot lump of 39.9 g of iron at an initial temperature of 78.1 °C is placed in 50.0 mL H 2 O initially at 25.0 °C and allowed to reach thermal equilibrium. What is the final temperature of the iron and water, given that the specific heat of iron is 0.449 J/(g⋅°C)? Assume no heat is lost to surroundings.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
A hot lump of 39.9 g of iron at an initial temperature of 78.1 °C is placed in 50.0 mL H 2 O initial...
Questions

Biology, 25.03.2020 05:10



Mathematics, 25.03.2020 05:10




Mathematics, 25.03.2020 05:10


Mathematics, 25.03.2020 05:10


Mathematics, 25.03.2020 05:10



Chemistry, 25.03.2020 05:10

History, 25.03.2020 05:10

Mathematics, 25.03.2020 05:10



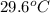
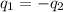
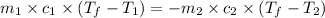
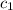 = specific heat of iron =
= specific heat of iron = 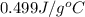
 = specific heat of water =
= specific heat of water = 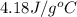
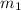 = mass of iron = 39.9 g
= mass of iron = 39.9 g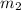 = mass of water =
= mass of water = 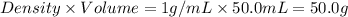
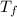 = final temperature of mixture = ?
= final temperature of mixture = ?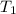 = initial temperature of iron =
= initial temperature of iron = 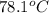
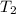 = initial temperature of water =
= initial temperature of water =