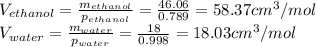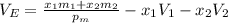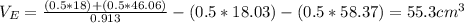Chemistry, 30.03.2020 19:16 hannah1571
The density of an equal-mass water 1 ethanol mixture is 0.913 g/cc at 20°C. If the density of water is 0.998 g/cm3 and ethanol is 0.789 g/cm3 with both at 20°C, does this equal-mass mixture possess a positive or negative excess volume at 20°C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
The density of an equal-mass water 1 ethanol mixture is 0.913 g/cc at 20°C. If the density of water...
Questions

Social Studies, 26.08.2019 11:30

Physics, 26.08.2019 11:30





History, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

History, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

Physics, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

History, 26.08.2019 11:30



Social Studies, 26.08.2019 11:30



Mathematics, 26.08.2019 11:30