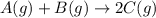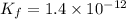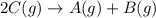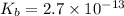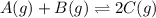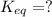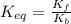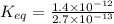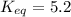Chemistry, 30.03.2020 19:28 starlightmoon213
Find the equilibrium constant for the reaction: A(g) + B(g) ⇌ 2C(g) at 25°C when k equals 1.4 × 10-12 M-1s-1 for the reaction: A(g) + B(g) → 2C(g) at 25°C and k equals 2.7 × 10-13 M-1s-1 for the reaction: 2C(g) → A(g) + B(g) at 25°C.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

You know the right answer?
Find the equilibrium constant for the reaction: A(g) + B(g) ⇌ 2C(g) at 25°C when k equals 1.4 × 10-1...
Questions

Computers and Technology, 31.01.2020 07:03


Chemistry, 31.01.2020 07:03


Mathematics, 31.01.2020 07:03


Mathematics, 31.01.2020 07:03


Mathematics, 31.01.2020 07:03



History, 31.01.2020 07:03


Mathematics, 31.01.2020 07:03



English, 31.01.2020 07:03



Biology, 31.01.2020 07:03