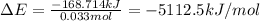Chemistry, 30.03.2020 19:25 hsjsjsjdjjd
When a 3.80 g sample of C8H18(l) is burned in a bomb calorimeter, the temperature of the calorimeter rises by 27.3 oC. The heat capacity of the calorimeter, measured in a separate experiment, is 6.18 kJ/oC. Determine the ΔE for C8H18(l) in units of kJ/ mole C8H18(l).

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3


Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
When a 3.80 g sample of C8H18(l) is burned in a bomb calorimeter, the temperature of the calorimeter...
Questions

Mathematics, 11.11.2019 23:31


Mathematics, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31

Chemistry, 11.11.2019 23:31


Mathematics, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31


Mathematics, 11.11.2019 23:31





Computers and Technology, 11.11.2019 23:31


Mathematics, 11.11.2019 23:31


Mathematics, 11.11.2019 23:31

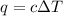
 = change in temperature = 27.3°C
= change in temperature = 27.3°C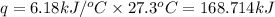
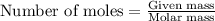
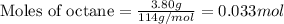
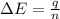
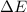 = enthalpy change of the reaction
= enthalpy change of the reaction