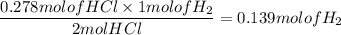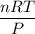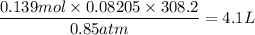Chemistry, 30.03.2020 19:29 dazesreplayy5363
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved. Calculate the volume(in L) of H2(g) at 35.2°C and 646 torr that can be formed when 325 mL of 0.855 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
Mg(s) + HCl(aq) --> MgCl2(aq) + H2(g) (unbalanced)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1



Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved. Calcula...
Questions



Mathematics, 19.01.2022 16:10





Social Studies, 19.01.2022 16:10

SAT, 19.01.2022 16:10



SAT, 19.01.2022 16:20



Physics, 19.01.2022 16:20

SAT, 19.01.2022 16:20




Mathematics, 19.01.2022 16:20