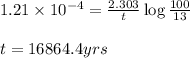Chemistry, 30.03.2020 20:15 buddyclayjohnson
Carbon-14 (14C) dating assumes that the carbon dioxide on the Earth today has the same radioactive content as it did centuries ago. If this is true, then the amount of 14C absorbed by a tree that grew several centuries ago should be the same as the amount of 14C absorbed by a similar tree today. A piece of ancient charcoal contains only 13% as much of the radioactive carbon as a piece of modern charcoal. How long ago was the tree burned to make the ancient charcoal? (The half-life of 14C is 5715 years. Round your answer to one decimal place.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Carbon-14 (14C) dating assumes that the carbon dioxide on the Earth today has the same radioactive c...
Questions

Mathematics, 23.08.2019 13:00





Mathematics, 23.08.2019 13:00





Advanced Placement (AP), 23.08.2019 13:00






Geography, 23.08.2019 13:00



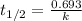
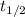 = half life of the reaction = 5715 years
= half life of the reaction = 5715 years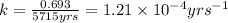
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0570/9587/f1041.png)
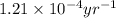
![[A_o]](/tpl/images/0570/9587/dc622.png) = initial amount of the sample = 100 grams
= initial amount of the sample = 100 grams