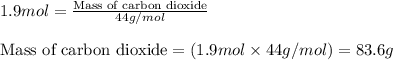Chemistry, 30.03.2020 22:16 janahiac09
Part B When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 22.8 g of carbon were burned in the presence of 73.8 g of oxygen, 13.0 g of oxygen remained unreacted. What mass of carbon dioxide was produced? Express your answer to one decimal place and include the appropriate units. View Available Hint(s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2


Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
Part B When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 22.8 g of ca...
Questions


Mathematics, 30.06.2019 15:00



Mathematics, 30.06.2019 15:00






Mathematics, 30.06.2019 15:00

Mathematics, 30.06.2019 15:00



Biology, 30.06.2019 15:00

Biology, 30.06.2019 15:00

Mathematics, 30.06.2019 15:00

Health, 30.06.2019 15:00


History, 30.06.2019 15:00

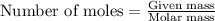 .....(1)
.....(1)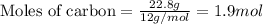
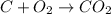
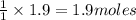 of carbon dioxide gas
of carbon dioxide gas