
Chemistry, 30.03.2020 20:24 jonmorton159
Consider the following reaction at 298 K. C ( graphite ) + 2 H 2 ( g ) ⟶ CH 4 ( g ) Δ H ∘ = − 74.6 kJ and Δ S ∘ = − 80.8 J / K Calculate the following quantities. Δ S sys = J/K Δ S surr = J/K Δ S univ = J/K Is this reaction spontaneous? yes no

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Consider the following reaction at 298 K. C ( graphite ) + 2 H 2 ( g ) ⟶ CH 4 ( g ) Δ H ∘ = − 74.6 k...
Questions











Mathematics, 06.11.2019 06:31

Chemistry, 06.11.2019 06:31


Mathematics, 06.11.2019 06:31


Physics, 06.11.2019 06:31

Mathematics, 06.11.2019 06:31

Biology, 06.11.2019 06:31

Advanced Placement (AP), 06.11.2019 06:31

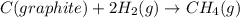
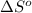 = Entropy of system = -80.8 J/K
= Entropy of system = -80.8 J/K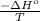
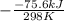
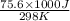
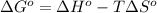
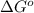 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?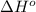 = standard enthalpy = -74600 J
= standard enthalpy = -74600 J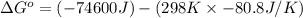
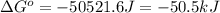
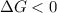 A reaction to be non-spontaneous when
A reaction to be non-spontaneous when 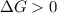
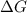 is negative or we can say that the value of
is negative or we can say that the value of 

